Normal and Abnormal First Trimester Exam
Normal First Trimester Exam
Page Links: Preconception, Chronology of Development, Ultrasound Findings, Early Gestational Sac, 5th Week, Decidua, Gestational Sac, Yolk Sac, Amnion and Chorion, MSD, Discriminatory hCG Levels, Mid-gut Herniation, Heart, Crown Rump Length (CRL), Nuchal Translucency (NT), International Standards for CRL and Gestational Age, Measurements and First Trimester Pregnancy Viability, References
Some commentary and images courtesy of Jill Beithon RT, RDMS, RDCS, RVT
Methods for Recommending Due Date
The American College of Obstetricians and Gynecologists (ACOG) (Committee Opinion 611, October 2014) along with the American Institute of Ultrasound in Medicine, and the Society for Maternal-Fetal Medicine have issued recommendations for estimating gestational age and due date.
In summary:
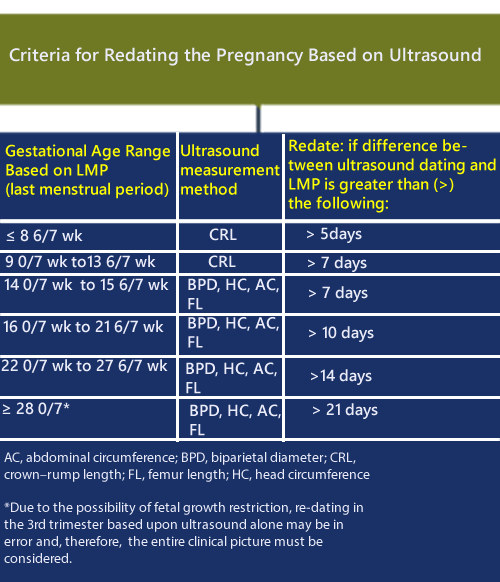
1. The most accurate method to establish or confirm gestational age is a CRL taken up to 13 6/7 weeks.
2. In the case of assisted reproduction, the age of the embryo and the date of transfer should be used.
3. The last menstrual period (LMP), and the first accurate ultrasound examination should be the basis for the expected due date (EDD), discussed with the patient and recorded in the medical record.
4. Using criteria in the document, the best obstetric estimate is recommended for the purposes of clinical care while the criteria for research and surveillance are presented.
The following chart summarizes the guidelines for “Re-dating the pregnancy on the basis of the ultrasound findings” when there is a difference between the ultrasound dates and the LMP (last menstrual period).
An update on methods for estimating due date is available here: Full Article (Updated 2017)
Due Date Calculator
A pregnancy due date and gestational age calculator is available here: http://perinatology.com/calculators/Due-Date.htm
First Trimester Ultrasound Scan
Preceded by pre-test counseling, women should be offered a first trimester ultrasound (US) (11 to 13 6/7 weeks) according to ISUOG recommendations if there are clinical concerns, pathological symptoms, or specific indications. The full text of this article is available and provides details for the performance of the first trimester fetal ultrasound scan. [1]
Above left. Maternal ovary. Approximately 24 hours before ovulation, a hypoechoic ring within the GF is seen (cumulus oophorus), which contains the oocyte.
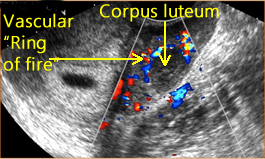
Above right. Maternal ovary. The corpus lutuem (CL) represents the ruptured GF, which is a thin-walled cyst with circumferential blood flow demonstrated by color Doppler. The CL secretes progesterone and a small amount of estrogen to stimulate endometrial proliferation.
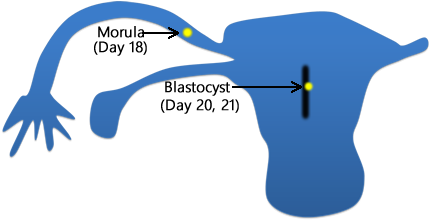
Above. After fertilization [2] the zygote undergoes rapid cell division and 3 days later, just before entering the uterus, the 12 to 16 cell stage (morula) is present. Within the uterus, the conceptus is termed the blastocyst.
Above. The blastocyst is composed of an outer cell layer, the trophoblast, which is destined to form part of the placenta and an inner cell layer, which is destined to form the embryo, amnion, umbilical cord, and yolk sac.
Chronology of Development
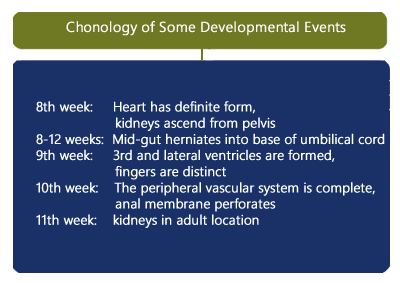
Above. The embryo undergoes some of the following developmental sequences. [3] By the 8th week, the heart has a definite form and by the 10th week, the peripheral vascular system is complete. By 8 to 12 weeks, the mid-gut herniates into the base of the umbilical cord. By the 9th week, the 3rd and lateral ventricles are formed, and the fingers are distinct. By the 10th week, the anal membrane perforates. The kidneys ascend from the pelvis in the 8th week and are in their adult location by the 11th week.
Ultrasound Findings
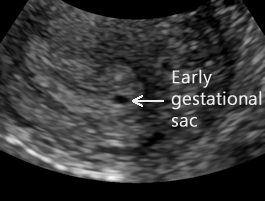
Early Gestational Sac
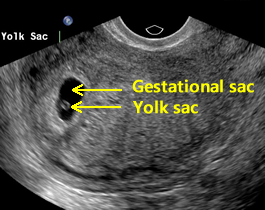
Above. The earliest sign of an intrauterine pregnancy (IUP) is a small gestational sac seen on transvaginal ultrasound at approximately 4 to 5 weeks and at that time, the mean sac diameter (MSD) is approximately 2.5 mm. The early sac should be round and adjacent to (not within) the linear interface of the endometrial lining, without displacing or deforming it.
This small round fluid collection (the chorionic cavity) is completely surrounded by a hyperechoic rim of tissue about 2 to 3 mm thick, which represents the developing chorionic villi and adjacent decidual tissue. The gestational sac is usually positioned in the mid to upper uterus.
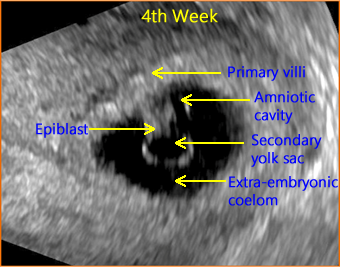
5th Week
Above. The gestational sac is now visible and contains the secondary yolk sac lying opposite the amniotic cavity with the embryonic disk (epiblast) between them. The epiblast will go on to form the definitive embryo and fetus. The extra-embryonic coelom is the subsequent chorionic cavity (the space between the amnion and chorion). By about 14 weeks, the chorionic cavity is normally obliterated and the amnion-chorion membrane forms the amniotic sac within which is the amniotic fluid (“bag of waters”).
Decidua
Above. By 5 to 6 weeks, two concentric echogenic lines surround a portion of the gestational sac and can be seen on trans-abdominal ultrasound.
Above. On transvaginal ultrasound, note the decidua capsularis and the decidua parietalis, findings which are sometimes referred to as the double decidual sign. Note the YS which is typically the first structure visualized in an IUP on transvaginal ultrasound.
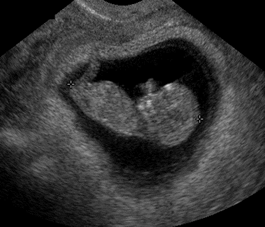
Gestational Sac
Above. On transvaginal ultrasound, a gestational sac is identified at 5 weeks. [4] In patients with accurate dates, cardiac motion is seen typically after 5 5/7 weeks, while a yolk sac is first seen at 5 1/7ths to 5 5/7ths weeks. [5] When an embryonic heartbeat is first seen, the MSD is 20 mm and at the time of embryo movements, the MSD reaches 30mm. [6]
Yolk Sac
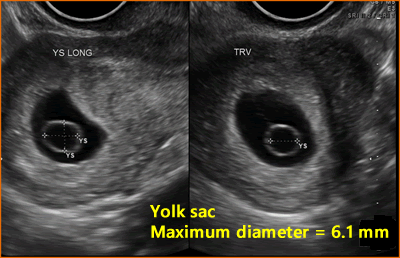
The yolk sac has an echogenic periphery with a sonolucent center. Detection of a yolk sac confirms that the intrauterine fluid represents an IUP and the number of yolk sacs usually correspond to the number of amnions. Yolk sac number is useful in assessing amnion number in multiple gestations. The diameter of the yolk sac increases between 5 to 10 weeks to a maximum of about 6mm, [7] and the measurement of the yolk sac is usually from inner wall to inner wall.
Amnion and Chorion
Above. The origin of the chorion is different from that of the amnion since the outer cell layer of the blastocyst forms the chorionic membranes and the inner cell layer forms the amnion. The chorion and amnion usually fuse by about 14 weeks. Typically, the amnion is visualized after the YS.
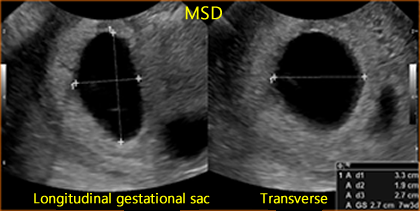
Mean Sac Diameter (MSD)
Above. The mean sac diameter (MSD) is usually 5 mm at by approximately 5 weeks. The MSD is calculated by adding 3 orthogonal dimensions of the chorionic cavity, excluding the hyper-echoic rim of tissue, and dividing by 3 (Length + Width + Height/3). MSD growth in normal gestation increases approximately 1.1 mm/day, while the embryo grows at 1 mm/day. [8]
Discriminatory hCG Levels
The discriminatory β-hCG level is the value above which an intrauterine gestational sac is seen on a consistent basis by ultrasound. This value is commonly cited as between 1000 and 2000 mIU per milliliter (World Health Organization 3rd or 4th International Standard). As noted by Doubilet and others, a single hCG level does not reliably distinguish among a normal intrauterine pregnancy, an ectopic pregnancy, or a nonviable pregnancy. [9] (Please see below).
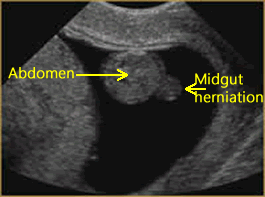
Mid-gut Herniation
Above. Physiological herniation of bowel into the base of the umbilical cord is part of the normal developmental sequence which occurs at about the 6th to 8th weeks, and by 8 to 12 weeks, the mid-gut herniates back into the base of the umbilical cord.
Heart
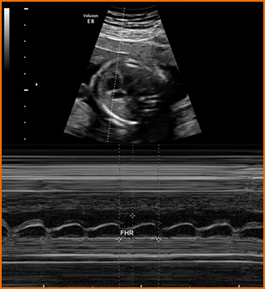
Above. M-mode embryonic heart. Embryonic and/or fetal cardiac activity should be documented. Utilize M-mode – not pulsed Doppler – for that purpose. With regards to ultrasonic exposure settings, use the ALARA principle (As Low As Reasonably Achievable). At < 6 weeks, the normal fetal heart rate is 100-115 beats per min (BPM). A rate of < 90 BPM is considered embryonic bradycardia.
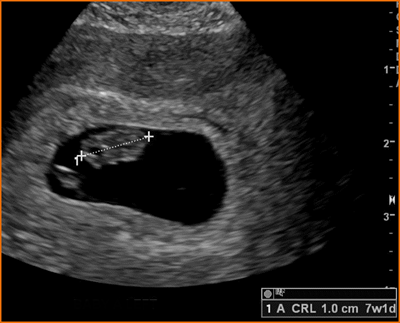
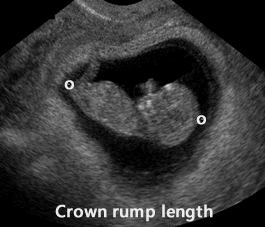
Crown Rump Length (CRL)
Above. Measured between 6 and 10 weeks, the CRL is the most accurate method for dating pregnancies. The longest axis of the embryo is measured and the yolk sac is identified separately from the embryo and is not included in the measurement.
Nuchal Translucency (NT)
Above. Normal NT.
Above. Increased NT.
The NT (See Genetic Markers) measures posterior neck nuchal fluid and a measurement of > 3 mm is always abnormal and is a marker for fetal aneuploidy and certain malformations, especially the heart.
The NT is measured at a gestation age of 11w0d to 13w6d weeks with a corresponding CRL of between 45 mm and 84 mm. A mid-sagittal view is taken in a neutral position away from the amnion. The maximum translucency is obtained with the callipers “on-to-on” as illustrated. A certification program is available through: The Fetal Medicine Foundation.
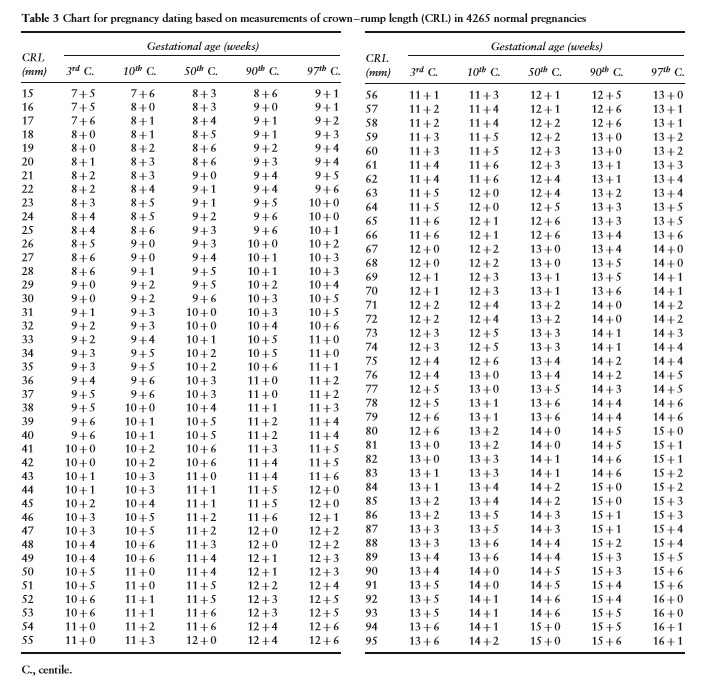
International Standards for CRL and Gestational Age
The following are charts from the open access article “International standards for early fetal size and pregnancy dating based on ultrasound measurement of crown-rump length in the first trimester of pregnancy.” [10] (This article is freely downloadable). The purpose of the article is to establish international standards for CRL in relationship to gestational age.

Above. Fetal crown-rump length (CRL) as a function of gestational age (GA). Gray open circles: raw data. Black open circles: empirical means.
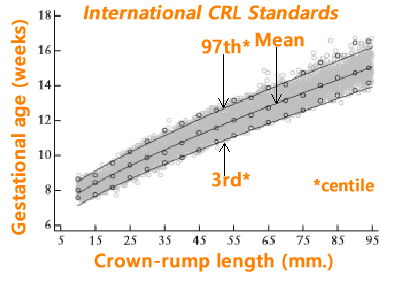
Above. Estimation of gestational age (GA) as a function of crown-rump length (CRL). Gray open circles: raw data. Black open circles: empirical means.
The following is another chart from the open access article.
A calculator is available on: Perinatology.com. If the CRL is known, the estimated gestational age and expected nuchal translucency can be obtained. If the NT measurement is known, the calculator will yield the NT percentile.
Measurements and First Trimester Pregnancy Viability
Measurements
Anatomy can be defined and some standard fetal measurements can be taken during the first trimester.

Above. BPD (biparietal diameter) and HC (head circumference) taken at 13 3/7 weeks.
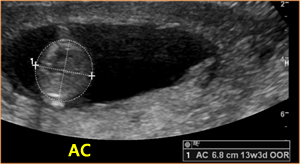
Above. AC (abdominal circumference) taken at 13 3/7 weeks.

Above. FL (femur length) taken at 13 3/7 weeks.
Pregnancy Viability
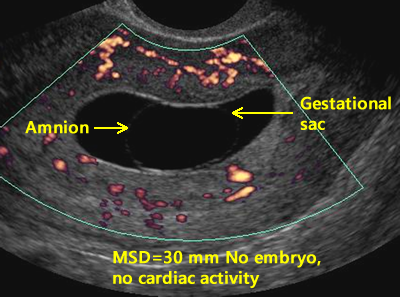
In order to avoid interruption of a normal pregnancy, criteria for a nonviable pregnancy includes the following in the presence of an empty sac: a mean sac diameter (MSD) of 25 mm or greater and a crown rump length of 7 mm or greater. In a retrospective review, Hu, Poder, and Filly determined that only 12% of 1013 threatened abortions met these present conservative guidelines for non-viability as recommended by the Society of Radiologist. [11] Clearly, there is much room for improvement.
An important task is to define normality in the first trimester by correctly assigning whether a pregnancy is viable, nonviable, or in an ectopic location. The false positive rate (the incorrect diagnosis of a nonviable pregnancy when the pregnancy is normal) can be as high as 4.4%. [12]
A recent review [13] defines pregnancy failure and specifies cutoff values for this purpose. Transvaginal ultrasound diagnosis of pregnancy failure in a pregnancy with uncertain viability is as follows:
CRL of ≥ 7 mm and no evidence of heartbeat.
MSD of ≥ 25 mm and no embryo visualized.
Initial scan shows a gestational sac without a yolk sac followed by a scan ≥ 2 weeks later demonstrating the absence of embryo with heartbeat.
Initial scan shows a gestational sac with a yolk sac followed by a scan ≥ 11 days later showing the absence of embryo with heartbeat.
If viability remains unclear, repeat transvaginal ultrasound in 11 to 14 days.
References
First Trimester: Bleeding, Miscarriage, and Molar Changes
Page Links: Normal, Threatened Pregnancy Failure, Criteria for a Non-Viable Pregnancy, First Trimester Bleeding, Miscarriage, Incomplete Miscarriage, Examples of First Trimester Bleeding, Complete Miscarriage, Gestational Trophoblastic Disease, References
Some commentary and images courtesy of Jill Beithon RT, RDMS, RDCS, RVT.
Normal
At 6 to 8 weeks gestation, normal ranges are established for crown-rump length (CRL), heart rate (HR), gestational sac diameter (GSD), and yolk sac diameter (YSD). [14]
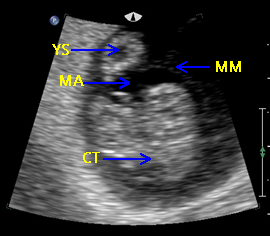
Above. Normal gestational sac with maximum YSD of 6.1 mm. Most recommend yolk sac measurement from inner wall to inner wall as demonstrated.
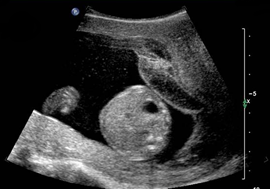
Above. Normal gestational sac with yolk sac, amnion, and embryo at about 6 menstrual weeks.
Above. Normal gestational sac and yolk sac at about 5 weeks.
Seen with transvaginal ultrasound (TVS) at about 5 weeks, the normal yolk sac (YS) is composed of 3 layers (ectodermal, mesodermal, and endodermal) and begins to regress after the 7th week, while the maximum diameter of the YS from 5 to 10 weeks is 5 mm to 6 mm. [15]
At 6 to 10 weeks, a deformed or absent yolk sac is not encountered in a normal pregnancy with a detected heartbeat. [16]
Normally, there is the sequential appearance of the yolk sac, HR, and amniotic membrane (amnion). The largest yolk sac in a continuing pregnancy is about 8.1 mm. In a first trimester pregnancy, the HR and YSD progressively increase. [17]
An intrauterine pregnancy (IUP) is typically diagnosed if there is a yolk sac, or a gestational sac with or without a fetal pole or a fetal pole with or without a HR.
Threatened Pregnancy Failure
Patients with threatened abortion may exhibit a normal YSD or abnormal YSD. 40% of patients with missed abortion will have an abnormal YSD outside of the 5th to 95th confidence interval. [18]
Significant differences exist in YSD between normal pregnancies and those with spontaneous miscarriage and threatened abortions at < 12 weeks. [19]
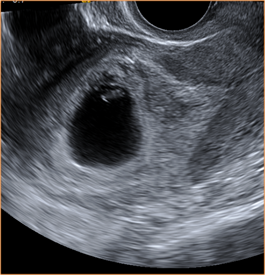
Above. In this case, no yolk sac is present and the MSD is ≥ 25 mm, while no embryo or cardiac activity is detected, meeting criteria for a non-viable pregnancy.
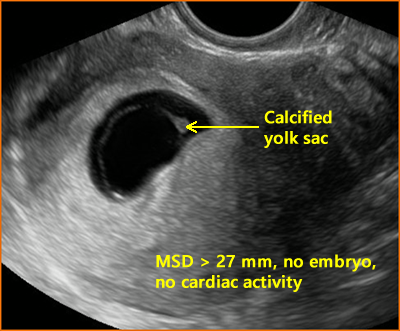
Above. In this case, the yolk sac (YS) is calcified and the MSD is ≥ 25 mm, while no embryo or cardiac activity is detected, again meeting criteria for a non-viable pregnancy.
In an embryonic gestation, the YS may be absent, irregular in shape, or relatively large. In a missed abortion with a prior HR, the YS may be relatively large, regressing, or deformed. [20]
In summary, the YS may be small, calcified, echogenic, irregularly shaped, or persistent in pregnancies destined for poor outcome, as noted in a sonographic pictorial of the YS. [21]
Termination of pregnancy should not be based on YS characteristics alone since a normal pregnancy may be ended. In a stable patient, follow-up ultrasound in 11 days to 2 weeks should be considered if other criteria for a non-viable pregnancy are not met.
Criteria for a Non-Viable Pregnancy
Using TVS, the following are criteria for a non-viable pregnancy [22]:
1. The absence of a heartbeat and the crown-rump length (CRL) is ≥ 7 mm.
2. The absence of the embryo and the MSD is ≥ 25 mm.
3. The initial scan shows a gestational sac without a yolk sac followed by a scan ≥ 2 weeks later demonstrating the absence of embryo with heartbeat.
4. The initial scan shows a gestational sac with a yolk sac followed by a scan ≥ 11 days later showing the absence of embryo with heartbeat.
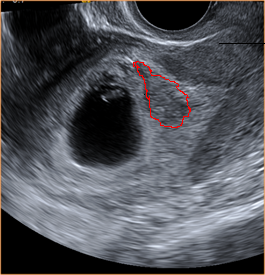
Above. The gestational sac is irregular and meets criteria for a non-viable pregnancy.
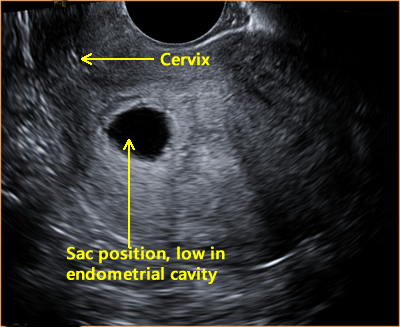
Above. While the sac position is low in the endometrial cavity, not all criteria are met for a non-viable pregnancy.
First Trimester Bleeding
About 20% of all pregnancies are associated with bleeding and pain during the first trimester, while most of these pregnancies will progress uneventfully. [23]
Bleeding may be due to threatened, incomplete, or spontaneous miscarriage; ectopic pregnancy or gestational trophoblastic disease. Genetic abnormalities account for 50% to 70% of spontaneous abortions, while 20% of patients with first trimester bleeding have a subchorionic bleed or hematoma. [24]
Another 10% to 15% of patients with first trimester bleeding will have an ectopic pregnancy. [25]
Miscarriage
Miscarriage is defined as the loss of a pregnancy prior to the completion of 24 weeks gestation. The main maternal symptoms are bleeding and pain. If a fetal HR has been detected, the risk of spontaneous miscarriage in singletons is 12.2%. [26]
In twin gestations, a significant CRL discrepancy at 7 weeks to 9 6/7 weeks is associated with the loss of one twin, while a discrepancy of <2 0% is associated with a 97% rate of viable twin pregnancies. [27]
A Randomized Trial of Progesterone in Women with Recurrent Miscarriages [28]
A multicenter, double-blind, placebo-controlled, randomized trial was undertaken in 836 women. The aim was to determine whether supplementation with progesterone in the first trimester of pregnancy would increase the rate of live births among women with a history of unexplained recurrent miscarriages. Women were randomly assigned to receive either twice-daily vaginal suppositories containing either 400 mg of micronized progesterone or matched placebo. In women with a history of unexplained recurrent miscarriages, progesterone therapy did not result in higher rates of live births.
Incomplete Miscarriage
Above. Incomplete miscarriage with “products of conception” (POC), which can include trophoblastic tissue, clot, and embryonic remnants.
Incomplete miscarriage occurs when there is partial passage of POC. Retained products remain within the endometrial cavity and the risk for continued maternal bleeding remains. TVS in the sagittal, transverse, and oblique planes can be used to assess the uterine contents. The presence of heterogeneous, irregular tissue with or without the presence of a gestational sac distorts the endometrial mid-line echo. [29]
Examples of First Trimester Bleeding
First trimester bleeding may be mild and insignificant or profuse. Resolution of subchorionic bleeding and clot is possible and observation of bleeding over time can change from acute, to chronic, to resolved.
Above. Note area of outlined bleeding, which is acute.
Above. Note area of chronic bleeding about 1 week after acute episode. Fetal cardiac activity and fetal growth are normal.
Above. Same patient, 2 weeks later. Image demonstrates resolution of subchorionic bleeding.
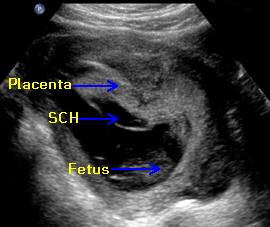
Above. 11 6/7 weeks gestation. Note the anterior placenta and the amnion chorion separation from the chorionic plate. The anechoic area represents fluid and or blood.
Above. First trimester SCH (subchorionic bleed). The relative positions of the amnion and chorion are illustrated. There is an extensive anechoic collection of fluid and blood in the subchorionic space.
Above. At the margin of the placenta, the amnion chorion membrane is elevated. The mixed echoes suggest clot between the amnion and chorion while there is fluid or blood in the subchorionic space and some evidence of marginal acute bleeding.
Above. Extensive hematoma in a woman with chronic bleeding. This represents a mixed pattern with anechoic areas suggesting fluid or blood and the mixed echos suggesting clot formation.
Above. Again, extensive hematoma in a woman with chronic bleeding. This represents a mixed pattern with anechoic areas suggesting fluid or blood and the mixed echos suggesting clot formation. In addition, the small echogenic particles representing blood fragments within the amniotic fluid are consistent with a history of maternal bleeding.
Blood clots may be present and are avascular and irregular on color Doppler. After confirmation of incomplete abortion on TVS, the patient may choose surgical evacuation or expectant management. Expectant management with close medical follow-up may be successful. In one study, 83% of women who chose expectant management spontaneously completed the process by week 2. [30]
Complete Miscarriage
A complete miscarriage occurs when the endometrial cavity is empty of the products of conception after a prior diagnosis of an IUP was established. A pregnancy of unknown location (PUL) occurs when there is no evidence for an IUP or ectopic pregnancy on TVS. A failing pregnancy can be suspected but not confirmed if the serum β-hCG declines 13% or more over a 48 hour period. [31]
On TVS, no evidence for products of conception are noted within the endometrial cavity in complete miscarriage. Scan planes are the same as those established for incomplete miscarriage.
Gestational Trophoblastic Disease
Gestational trophoblastic neoplasia (GTN) terminology includes complete hydatidiform mole (CHM), partial hydatidiform mole (PHM), invasive mole, choriocarcinoma, and placental site trophoblastic tumor. These neoplastic changes in placental trophoblastic tissue are typically associated with increased hCG production and may have other clinical manifestations such as early onset preeclampsia, hyperemesis, and bleeding. Among the GTN subtypes, HM is the most common occurring at a frequency of 1 in 750 pregnancies. [32]
In CHM, there is generalized trophoblastic hyperplasia and swelling of the chorionic villi in the absence of an embryo or fetus, while in PHM, there is focal trophoblastic hyperplasia and focal swelling of villous tissue in the presence of fetal or embryonic tissue. [33]
Complete moles are paternally derived and partial moles are usually triploid.
Above. Placental triploidy. Note multiple clusters of grape-like cysts. The ultrasound appearance is “snow storm,” but this finding is less likely in the first trimester. (Images on triploidy are courtesy of Jane JK Burns, AS, RDMS).
There has been an evolution in the presentation of HM since the widespread introduction of obstetrical ultrasound. With routine first trimester ultrasound, 41% of the patients with CHM are asymptomatic. [34] Most HM pregnancies are evacuated during the first trimester and both the ultrasound features and the histopathology of the specimens are different compared to mid-trimester late diagnosis of HM. A majority of histologically proven HM are associated with a sonographic diagnosis of missed abortion or anembryonic pregnancy. [35]
Above. Note first trimester molar changes.
First trimester molar pregnancies may demonstrate cystic changes or increased placental echos with a diagnosis of CHM more likely at ≥ 13 weeks, but only 50% of the cases were diagnosed before 13 weeks. [36] Molar pregnancies have been diagnosed in 88% of CHM [37], and in a review of 16 patients, only 9 (56%) demonstrated the classic “snow storm” appearance. [38]
In a retrospective review of 1053 cases of partial or complete HM, a pre-evacuation diagnosis was made in 44% of cases with a final diagnosis of HM. Partial moles were detected less often compared with complete moles. [39]
Jauniaux and Nicolaides reported that 10 of 11 patients with either CHM or PHM demonstrated sonographic placental changes at 10 to 14 weeks, which included diffuse cystic lesions and multicystic ovaries in all patients with a β-hCG of > 8 MoM. [40] As suggested by these authors, cytogenetic investigation should be offered, while Β-hCG measurements and Doppler ultrasound measurements of the uterine artery may improve diagnostic potential. Routine histological examination of the products of conception is warranted as well as a follow-up regimen for the detection of persistent trophoblastic neoplasia.
References
First Trimester: Detection of Abnormalities
Page Links: Introduction, Overall First Trimester Detection, NT and Non-Chromosomal Abnormalities, NT and Adverse Pregnancy Outcomes, First Trimester Non-Chromosomal Abnormalities, NT and Congenital Heart Disease, CHD in High Risk Groups, Central Nervous System (CNS) Malformations, Kidneys, Abdomen, Other Abnormalities, References
Introduction
The detection of fetal anomalies in the first trimester is made possible with the advent of high-frequency transvaginal probes, which has resulted in defining a number of malformations and clarifying the optimal gestational age to visualize anatomical structures. [41] Systematic screening during the late first trimester (11 to 14 week scan) yields the greatest opportunity to define certain abnormalities [42], while there remains a need to evaluate and further define the effectiveness and place for early fetal assessment. [43]
Overall First Trimester Detection
In a prospective study of 1148 singleton pregnancies at 11 to 14 weeks, 50% of major structural malformations were detected, while a 2-stage process with a second mid-trimester scan detected 42.8% of abnormalities for a total detection rate of 92.8%. [44] Complete fetal anatomy was seen in 33% of cases at 11 to 13 6/7 weeks in one study, while the cranium, intracranial anatomy, face, cord insertion, stomach, and 4 limbs were seen in 95% of cases and fetal heart in 84%. [45] In a systematic review encompassing 19 studies, the average sensitivity for first trimester detection of abnormalities was 50%. [46] In the future, 3-dimensional virtual reality ultrasound may increase the first trimester detection rate for certain malformations such as those of the skeleton and skin. [47] Due to the late development of some organs as well as the developmental sequence of malformations, first trimester ultrasound will not be able to detect all defects. [48]
NT and Non-Chromosomal Abnormalities
NT is a fluid filled subcutaneous space in the posterior neck region that can be reliably measured from 10 weeks 3 days to 13 weeks 6 days gestational age.
NT and Adverse Pregnancy Outcomes
In addition to its strong association with fetal aneuploidies, increased NT is linked to the risk of adverse perinatal outcomes including fetal malformations, dysplasia, deformations, disruptions, and genetic syndromes, but these risks do not reach significance until the NT is ≥ 3.5 mm (> 99th percentile). [49] The fetal death rate depends upon the degree of abnormality of the NT (at 3.5-4.4 mm, death rate = 2.7%, at >6.5 mm, death rate = 19%). [50] Alternatively, if the fetus survives to the mid-trimester and no abnormalities are noted on the targeted scan, the risk of adverse outcome does not significantly increase. [51]
Specialized training and certification for sonographers is required for the NT measurement. A certification program is available through The Fetal Medicine Foundation.
A calculator is available on Perinatology.com. If the CRL is known, the estimated gestational age and expected nuchal translucency can be obtained. If the NT measurement is known, the calculator will yield the NT percentile.
First Trimester Non-Chromosomal Abnormalities
The first trimester examination offers additional benefits for confirming gestational age and viability, identifying other structural malformations, and suggesting potential pregnancy complications (stillbirths, preterm delivery, preeclampsia, gestational diabetes, and fetal macrosomia). [52]
In fetuses without chromosomal anomalies, certain malformations are commonly detected at the 11 to 14 week scan, especially those of the CNS (acrania, alobar holoprosencephaly, and exomphalos) and of the abdomen (gastroschisis, megacystis, and body stalk anomaly), while others are less commonly defined (50% diaphragmatic hernia and 50% lethal skeletal dysplasias). [53] In the same study, non-detected malformations included agenesis of the corpus callosum, cerebellar hypoplasia, echogenic lung lesions, bowel obstruction, and renal defects.
NT and Congenital Heart Disease
In fetuses without chromosomal abnormalities, the incidence of congenital heart disease is increased in those with an NT value above the 95th percentile, which is present in approximately one-third of fetuses with major cardiac defects. [54] The risk is dependent upon the degree of NT abnormality and when the NT is ≥ 3.5 mm, heart defects are present in 1 of 43 singleton pregnancies. [55]
Goetzl has reviewed the literature on this topic [56] and when the NT is ≥ 3.5 mm, the incidence of cardiac abnormalities is 6.0% (5.2-6.8%); when the NT is 3.5 to 4.4 mm, the incidence is 3.2% (2.3-4.1%); and when the NT is > 4.5 mm, the incidence is 11.8% (9.8 to 13.8%).
Congenital Heart Defects (CHD) in High Risk Groups
A combination of increased NT and the observations of tricuspid regurgitation as well as reversal of the “a” wave in the ductus venosus is predictive of major cardiac defects. [57] When the NT is above the 99th percentile or the ductus venosus a-wave is absent or reversed, a 47% detection rate for major cardiac defects is reported with a 2.7% false positive rate. [58]
Tricuspid regurgitation (TR) observed in the first trimester is present in approximately 7% of normal fetuses and has a significant association with fetal aneuploidy, especially in the presence of other markers but as an isolated finding, TR demonstrates a low screening potential for fetal aneuploidy and congenital heart defects. [59]
With qualified personnel and equipment, detection of major CHD in high-risk groups (TR, increased NT, abnormal DV flow) is reported with a sensitivity of 78.5% in the first and early second trimester.
Finally, fetal echocardiography in the first trimester has limitations and assessment of the fetal heart at this gestational age requires a high level of expertise and caution is advised. [60] In one review, the false positive rate is reported as high as 33% [61], while in expert hands the false positive rate is much lower.
Central Nervous System (CNS) Malformations
A number of structural CNS malformations are seen during the first trimester and include [62]: acrania anencephaly sequence, holoprosencephaly, cerebellar defects, hydranencephaly, encephalocele, and spina bifida. Enlargement of intracranial translucency and enlargement of the brain stem to occipital bone are potential markers for posterior fossa abnormalities during the first trimester. [63] Sonographers can be trained to perform intracranial translucency measurements at the 11 to 13 week exam, resulting in reproducible results. [64] In the detection of spina bifida in the first trimester, the non-visualization of the posterior fossa (cisterna magna) is reported as the best screening method. [65]
Kidneys
During the first trimester, the bladder is normally visualized in about 90% of cases. [66] Among fetuses with megacystis, about 38% were detected during the first trimester. The main etiologies were urethral occlusion and prune-belly syndrome, both of which carry a poor prognosis. [67]
Abdomen
Among abdominal defects, anterior abdominal wall defects are possible to detect during the first trimester. Body stalk abnormality is characterized by a malformed fetus with an anterior abdominal wall defect and a portion of the fetal body attached to the placenta, while a key finding is angulation of the fetal spine and kyphoscoliosis. Omphalocele occurs when there is herniation of the fetal bowel beyond 12 weeks gestation with cord insertion at the apex of the herniated sac. Gastroschisis is an anterior abdominal wall defect in which bowel herniates into the amniotic cavity with the cord insertion lateral to the defect.
Other Abnormalities
Other potential abnormalities seen in the first trimester are: cleft lip, diaphragmatic hernia with stomach visualized in the chest and mediastinal shift, and skeletal dysplasia with abnormal long bones, contractures, and altered echogenicity. [68]
References
First Trimester: Normal and Abnormal Images
Some commentary and images courtesy of Jill Beithon RT, RDMS, RDCS, RVT
Normal First Trimester Images
Above. Crown rump length, 11 weeks.
Above. The rhombencephalon is normally visualized between 7 and 9 weeks.
Above. Note embryonic rhombencephalon and fetal extremities.
Above. Axial view. Fetal skull and brain, 12 weeks.
Above. Sagittal view, 11 weeks. Note upper and lower extremities.
Above. Fetal hand, 13 weeks.
Above. Fetal lower extremities, 13 weeks.
Above. 4 chamber cardiac view, 13 weeks. Caution: Color Doppler in the first trimester should be used only when the benefit exceeds the risk.
Above. Stomach and umbilical cord, 13 weeks.
Above. Umbilical arteries and bladder, 13 weeks.
Above. Umbilical cord placental insertion, 13 weeks.
Abnormal First Trimester Images
Above. Abnormal NT.
Above. Anencephaly.
Above. 3-D. Anencephaly.
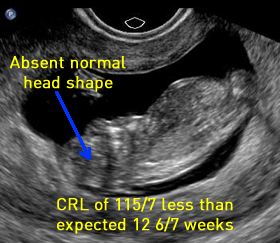
Above. Exencephaly 12 2/7 weeks. Neural tissue is still present during the first trimester, and the skull configuration is abnormal, while the the prominent orbits noted later are just appearing. CRL measures approximately 1 week less than expected for gestational age.
Above. Exencephaly 12 6/7 weeks. The correct designation is exencephaly since neural tissue is still present. Characteristics include less than expected gestational age measurements. Views demonstrating the absence of the cranial vault are possible at this gestational age. During the mid-trimester, little or no neural tissue is noted above the orbital ridge, and the irregular appearing “cerebrovasculosa” is visible.
Above. Cystic hygroma.
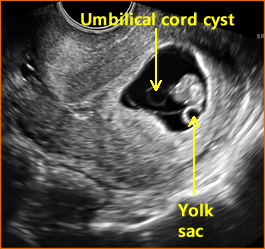
Above. Umbilical cord cyst.
Conjoined Twins: First Trimester
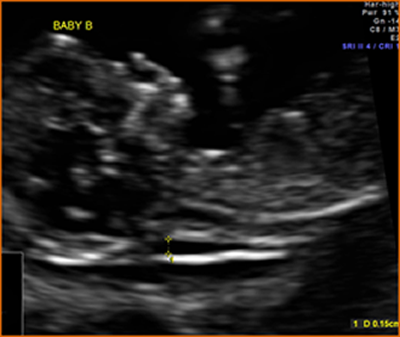
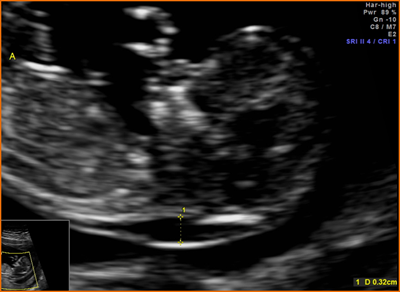
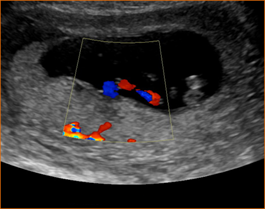
Above. Patient 1. Possible conjoined twins. Transvaginal ultrasound, 8 4/7 weeks. Brief color Doppler and subsequent M-mode demonstrate cardiac activity in each twin. Note the following conditions for conjoined twins: monoamniotic monochorionic membrane and monochorionic sac. This image is not definitive for conjoined twins.
Above. Patient 1. Probable conjoined twins. Transvaginal ultrasound, 8 4/7 weeks. Note that the following conditions for conjoined twins are present: monoamniotic monochorionic membrane and monochorionic sac. The twins appear conjoined, and manipulation of the uterus with the transvaginal probe does not alter their fixed relationship. False positives are possible in the first trimester, and a follow-up scan is warranted.
Above. Patient 2. Probable conjoined twins. Again, note conditions for conjoined twins are present: monoamniotic monochorionic membrane and monochorionic sac with close approximation of the fetuses. The twins appear conjoined and manipulation of the uterus does not alter their fixed relationship. Again, false positives are possible in the first trimester and follow-up scan is warranted.
Above. Patient 2. Probable conjoined twins. Again, note that the following conditions for conjoined twins are present: monoamniotic monochorionic membrane and monochorionic sac. The twins appear conjoined in this 3-D reconstructed view.
First Trimester: Ectopic Pregnancy
Page Links: Introduction, Ectopic Pregnancy (Tubal), Ectopic Pregnancy (Non-Tubal), Interstitial Pregnancy, Cornual Pregnancy, Cervical Pregnancy, Ovarian Pregnancy, References
Some commentary and images courtesy of Jill Beithon RT, RDMS, RDCS, RVT.
Introduction
Any gestation established outside the intrauterine cavity is considered an ectopic pregnancy (EP) and the incidence is 1% to 2% of pregnant women. [69] Ectopic pregnancies can be broadly classified as tubal, within the fallopian tube (FT), and non-tubal, which occur outside of the endometrial cavity but within the uterine wall or the cervix and account for 5% to 7% of all EPs. [70]
Above. Location of tubal and non-tubal ectopic pregnancies. Most EPs occur in the ampulla or isthmus of the FT.
Ectopic Pregnancy: Tubal
The classic triad for ectopic pregnancies is a pregnancy presenting with pain, bleeding, and the presence of a mass. Risk factors, which are reproductive failures, include history of tubal disease and smoking, which may help to establish a diagnostic and management strategy. [71] Other risk factors include, uterine anomalies, history of assisted reproduction, and pelvic infection.
On transvaginal ultrasound (TVS), a tubal ectopic pregnancy may be present if no intrauterine pregnancy (IUP) is seen. A definitive gestational sac with a yolk sac excludes the diagnosis, but fluid within the endometrial cavity in the absence of a YS does not exclude the diagnosis. Rarely, a heterotopic gestation may be present with one gestational sac consistent with an IUP and another simultaneous pregnancy which is ectopic in location. To confirm the likelihood of an tubal EP, one of the following conditions should be met [72]:
1. An inhomogeneous adnexal mass separate from the ovary.
2. An empty extra-uterine gestational sac with a hyperechoic ring in the adnexal region.
3. An extrauterine sac in the adnexal region with a yolk sac or fetal pole ± cardiac activity.
Above. Tubal ectopic pregnancy. Note empty gestational sac surrounded by increased vascularity in the adnexa region.
Above. Tubal ectopic pregnancy. Note inhomogeneous mass surrounded by increased vascularity in the left adnexa.
A pregnancy diagnosis is sometimes made but the location of the pregnancy is not readily apparent. In 2011, a consensus statement of definitions and outcomes for pregnancy of unknown location (PUL) was published. [73]
The following categories for ultrasound diagnosis were suggested:
1. Definite EP: Extrauterine gestational sac with yolk sac and/or embryo (with or without cardiac activity).
2. Probable EP: Inhomogeneous adnexal mass or extrauterine sac-like structure.
3. PUL: No signs of either EP or IUP.
4. Probable IUP: Intrauterine echogenic sac-like structure.
5. Definite IUP: Intrauterine gestational sac with yolk sac and/or embryo (with or without cardiac activity).
Treatment for tubal EP is either medical with methotrexate, or surgical by laparoscopy. Many protocols suggest periodic hCG measurements and transvaginal ultrasound.
Some treatment protocols recommend medical management in patients with suspected ectopic pregnancy if the β-hCG level is <1 000 IU/l, the patient is asymptomatic, and the EP is not visualized. However, a single β-hCG level cannot exclude the possibility of an intrauterine pregnancy, and despite numerous studies and meta-analysis, no single protocol or method of management has been established. At least 6 diagnostic protocols have been defined using a combination of ultrasound, β-hCG, and serum progesterone. [74] Finally, serum β-hCG may not be useful in distinguishing IUPs from ETs in symptomatic pregnant women. [75]
The following observations relate to treatment and recurrence risk [76]:
1. Irrespective of treatment mode, the chances of a subsequent successful IUP in EP patients are the same.
2. Generally, the size of the EP should not be a limiting factors for conservative laparoscopic surgery.
3. The risk of recurrent EP is about 10% after salpingectomy.
Ectopic Pregnancy: Non-Tubal
Ectopic pregnancies, which occur in locations other than the fallopian tube, are characterized by delayed diagnosis and increased morbidity compared with tubal EPs. These pregnancies include interstitial pregnancy (implantation in the interstitial portion of the FT), cornual pregnancy (rudimentary horn of unicornuate uterus), cervical pregnancy (and caesarean scar pregnancy), and ovarian pregnancy.
Interstitial Pregnancy
An interstitial pregnancy (IP) is implanted near the uterine cavity in the interstitial portion of the fallopian tube (the portion of the FT which is within the uterine wall and connects the FT to the uterine cavity). An IP is difficult to distinguish from an IUP which is implanted high and laterally in the uterine cavity. Under these conditions the coronal plane best demonstrates the gestational sac position, and 3-D ultrasound may be helpful. [77] On ultrasound, the gestational sac is surrounded by myometrium, which is capable of expanding and thus, delaying the interval from conception to rupture. The overall mortality is about 2% (1 in 50). [78]
Above. Probable interstitial pregnancy. Note gestational sac in anatomic region where FT crosses the uterine fundus. Within the gestational sac is a small YS and fetal pole. A portion of the sac is surrounded by myometrium.
Above. Interstitial pregnancy. 3-D image.
The ultrasound findings in an interstitial pregnancy usually include a normal uterine shape and normal uterine cavity with a narrow communication between the gestational sac and uterine cavity, while a myometrial panel which is continuous with the uterus is present, and there is no sac mobility or vascular pedicle. [79]
Cornual Pregnancy
A cornual pregnancy is defined as a gestation which implants in the rudimentary horn of a unicornuate uterus. A unicornuate uterus is a developmental abnormality of the Mullerian duct, where 1 horn instead of 2 uterine horns develop. However, the ectopic pregnancy develops in the opposite horn that is small and fails to fully develop (the rudimentary horn). This anomaly represents < 1% of congenital uterine anomalies in the general (fertile) population. [80]
Above. Approximate location of rudimentary horn of unicornuate uterus, which is the most likely location for a cornual pregnancy. (Image courtesy of RadsWiki, Creative Commons Attribution-Share Alike 3.0).
The following ultrasound criteria are necessary for the diagnosis of cornual pregnancy [81]:
1. A single interstitial portion of the fallopian tube in the main uterine body is affected.
2. A gestational sac surrounded by myometrium is mobile and separate from the uterus.
3. A vascular pedicle joins the gestational sac to the unicornuate (developed horn) of the uterus.
Above. Possible cornual pregnancy. The location is near the cornual region of the cervix. Diagnosis or confirmation of rudimentary horn is not certain in this case. Interstitial pregnancy remains a possibility.
Above. Same patient as immediately above. Note connecting stalk or pedicle.
The diagnosis is based upon finding a single interstitial tube, which is near an empty uterus. In a normal intrauterine pregnancy, there is a wide communication between the uterine cavity and the gestational sac, which is not the case in ectopic pregnancies. Other distinguishing features in a cornual pregnancy include the mobility of the gestational sac and the presence of a vascular pedicle.
Sensitivity is 26% for the ultrasound diagnosis of rudimentary horn, while the sensitivity is 14% for the diagnosis before clinical symptoms. [82]
If the diagnosis is made before rupture, surgical management is recommended either by laparoscopy (usually first trimester) or in advanced cases, open surgery. [83]
Cervical Pregnancy
A cervical ectopic is one in which the implantation occurs below the internal cervical os. The prevalence is 1:10,000 deliveries, while previous curettage (50% of cases) and cesarean delivery (16.7% of cases) are historical antecedents. [84] Other historical factors include uterine manipulation from hysteroscopy and history of in vitro fertilization, while clinical symptoms include vaginal bleeding and abdominal discomfort. [85]
The following criteria is suggested for the diagnosis of cervical pregnancy [86]:
1. Absence of gestational sac, fetal pole, or yolk sac within the endometrial cavity.
2. Hour glass uterine shape.
3. The presence of a gestational sac within the cervical canal.
4. Closed internal os.
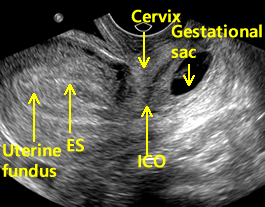
Above. Cervical pregnancy. Longitudinal scan. Note uterine fundus and ES (endometrial stripe). The ICO (internal cervical os) is closed and demarcates the cervix and the gestational sac is seen within the substance of the cervix.
Above. Cervical pregnancy demonstrating the enlarged cervix and portions of the gestational sac.
Above. Cervical pregnancy. Post medical injection. Note the absence of a gestational sac within the EC (endometrial cavity). The general region of the IO is demonstrated. Trophoblastic tissue is noted within the endocervical canal and the cervix appears enlarged.
Above. Cervical pregnancy. Same patient as immediately above demonstrating trophoblastic tissue in the endocervical canal.
Above video. Cervical pregnancy. Note the decidual changes within the endometrium in the fundus of the uterus. The cervical ectopic demonstrates a small fetal pole.
Above. Cervical pregnancy. Same as above. Note endometrial changes, and cervix with ectopic pregnancy.
Incomplete abortion versus cervical pregnancy
An incomplete abortion may have tissue within the cervix, while the internal os may be open if there is a large cervical pregnancy. The level of the internal os can be determined on transvaginal ultrasound by noting the insertion of the uterine arteries with color Doppler, which are at the level of the internal os. [87] Color Doppler will also illustrate the vascular supply to the trophoblastic tissue, while in incomplete abortions, the trophoblastic blood supply is disrupted. An incomplete abortion may be distinguished from a cervical pregnancy by the “sliding sign” in which a gestational sac from a detached IUP slides towards the cervical canal with uterine pressure on the intravaginal probe.
Management of cervical pregnancy
In a review of the management of cervical pregnancies, the following is suggested [88]:
1. Primary medical management of early cervical pregnancy is associated with less hemorrhage and fewer hysterectomies compared with surgical management.
2. Non-viable cervical pregnancies can be treated with systemic methotrexate.
3. Viable cervical pregnancies can be treated with local injections of methotrexate or potassium chloride.
4. Surgery should be reserved for failed medical management or patients presenting with catastrophic hemorrhage.
5. Surgical options include dilatation and curettage with cerclage or insertion of a Foley catheter to control hemorrhage.
Ovarian Pregnancy
Ovarian ectopic pregnancies are rare, comprising 2% of all EPs, while associated factors include assisted reproductive technology (ART) procedures (18.1%) and the presence of an intrauterine device (IUD) (19.4%). [89], [90] The presenting symptoms are similar to tubal EP and include bleeding and pain with circulatory collapse reported in 21% of cases in some series. [91]
On transvaginal ultrasound, a gestational sac is surrounded by normal ovarian tissue, while gentle palpation during the exam does not separate the sac from the surrounding ovary. Color Doppler is important in the assessment. [92] Two areas of vascularity may be seen within the ovary: the “ring of fire” surrounding the corpus luteum, and the increased vascularity due to the trophoblastic proliferation, which is typically thick and echogenic.
In a recent review, 52% of ovarian EPs were managed laparoscopically, while the success rate for medical management was 50% in a small number of cases. [93] Laparoscopy is used as a diagnostic and treatment method (wedge resection). [94]
References
-
Abstract: PMID: 23280739 Moore, KL, Persaud TVN, Shiota, K. In Color Atlas of Clinical Embryology. WB Saunders Company; 1994; p.1. Rumack, CM, Wilson SR, Charboneau, JW. In: Diagnostic ultrasound. Mosby, St. Louis, 1998, p 977. -
Abstract: PMID: 2030862 -
Abstract: PMID: 2660539 -
Abstract: PMID: 2030862 Rumack, CM, Wilson SR, Charboneau, JW. In: Diagnostic ultrasound. Mosby, St. Louis, 1998, p 997. Rumack, CM, Wilson SR, Charboneau, JW. In: Diagnostic ultrasound. Mosby, St. Louis, 1998, p 990. -
Abstract: PMID: 24106937 -
Abstract: PMID: 25044000 -
Abstract: PMID: 25154939 -
Abstract: PMID: 21997898 -
Abstract: PMID: 24106937 -
Abstract: PMID: 20847544 -
Abstract: PMID: 22215774 -
Abstract: PMID: 16953856 -
Abstract: PMID: 17693010 -
Abstract: PMID: 9435738 -
Abstract: PMID: 24779249 -
Abstract: PMID: 16953856 -
Abstract: PMID: 22215774 -
Abstract: PMID: 24106937 -
Abstract: PMID: 24841987 -
Abstract: PMID: 15905817 -
Abstract: PMID: 24841987 -
Abstract: PMID: 12871890 -
Abstract: PMID: 19521968 -
Abstract: PMID: 26605928 -
Abstract: PMID: 12099260 -
Abstract: PMID: 12099260 -
Abstract: PMID: 17468257 -
Abstract: PMID: 2414703 -
Abstract: PMID: 9060124 -
Abstract: PMID: 10985617 -
Abstract: PMID: 15846756 -
Abstract: PMID: 10478967 -
Abstract: PMID: 10985617 -
Abstract: PMID: 10478967 -
Abstract: PMID: 16273594 -
Abstract: PMID: 9060124 -
Abstract: PMID: 24176153 -
Abstract: PMID: 24355991 -
Abstract: PMID: 19155913 -
Abstract: PMID: 16458635 -
Abstract: PMID: 16157148 -
Abstract: PMID: 24216159 -
Abstract: PMID: 24440996 -
Abstract: PMID: 24201688 -
Abstract: PMID: 15846173 -
Abstract: PMID: 23814750 -
Abstract: PMID: 15846173 -
Abstract: PMID: 21210474 -
Abstract: PMID: 21210483 -
Abstract: PMID: 21210483 -
Abstract: PMID: 15902108 -
Abstract: PMID: 20638576 -
Abstract: PMID: 22482746 -
Abstract: PMID: 23152003 -
Abstract: PMID: 24799402 -
Abstract: PMID: 23751926 -
Abstract: PMID: 20638576 -
Abstract: PMID: 20638576 -
Abstract: PMID: 24065268 -
Abstract: PMID: 25063402 -
Abstract: PMID: 23494913 -
Abstract: PMID: 16157148 -
Abstract: PMID: 24745800 -
Abstract: PMID: 20638576 -
Abstract: PMID: 19625718 -
Abstract: PMID: 24841987 -
Abstract: PMID: 15388673 -
Abstract: PMID: 17468257 -
Abstract: PMID: 20947073 -
Abstract: PMID: 15388673 -
Abstract: PMID: 21310509 -
Abstract: PMID: 15388673 -
Abstract: PMID: 11876800 -
Abstract: PMID: 10438980 -
Abstract: PMID: 17763478 -
Abstract: PMID: 18539641 -
Abstract: PMID: 17763478 -
Abstract: PMID: 15932844 -
Abstract: PMID: 17763478 -
Abstract: PMID: 17630167 -
Abstract: PMID: 24976825 -
Abstract: PMID: 3314739 -
Abstract: PMID: 8141192 -
Abstract: PMID: 9014275 -
Abstract: PMID: 22921271 -
Abstract: PMID: 22663322 -
Abstract: PMID: 15099878 -
Abstract: PMID: 24841987 -
Abstract: PMID: 22663322 -
Abstract: PMID: 15200773
2
3
7
8