Gastroschisis
Gastroschisis
Page Links: Definition, Other Anterior Abdominal Defects, Prevalence, Classification, Etiology, Genetics, Risk Factors, Outcome, Sonographic Detection of Growth Restriction in Gastroschisis., Management, Route of Delivery for Infants with Gastroschisis, Fetal Testing of the Abdomen, References
Definition
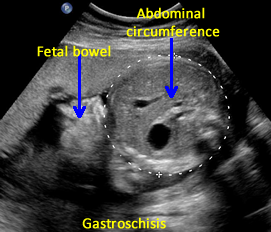
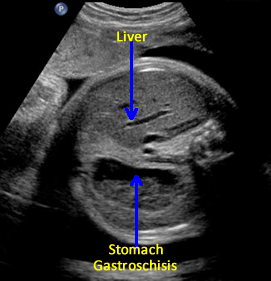
Gastroschisis is a defect in the anterior (ventral, front) wall of the fetal abdomen, which allows a variable amount of fetal bowel and other intra-abdominal contents to herniate through the defect into the amniotic fluid and the amniotic sac. [1] The umbilical cord inserts into its usual location. In addition, the fetal bowel or other contents are uncovered by membrane and the defect is usually to the right of the umbilicus.
Other Anterior Abdominal Defects
An omphalocele is a central midline anterior abdominal wall defect and is differentiated from gastroschisis by:
1. Its covered with a membranous sac.
2. Its location at the base of the umbilical cord. [2]
Other congenital abdominal wall defects include body stalk anomaly, prune-belly syndrome, pentalogy of Cantrell, amniotic band syndrome, bladder and cloacal exstrophy, and umbilical hernias. [3]
Body stalk anomaly may be lethal and is characterized by the attachment of fetal bowel and internal fetal organs to the placenta. Prune-belly infants have a thin, relaxed protruding anterior abdominal wall due to a lack of abdominal muscles and fetal urinary tract abnormality. Pentalogy of Cantrell and other defects are described separately in this section.
Prevalence
| Gastroschisis prevalence | Year |
| .1 per 10,000 | 1970s |
| 5 per 10,000 | 2000s |
Two factors have been observed over the past several decades: the prevalence of gastroschisis is increasing and lower maternal age for the defect is documented.
Above. The worldwide prevalence for gastroschisis is increasing and was 0.1 per 10,000 in the 1970s, but in the early 2000s, the prevalence increased to 5 per 10,000 births. [4] Overall, the ratio of omphalocele to gastroschisis is at a 3 to 2 ratio. [5]
In a study of 265,858 patients, the prevalence of gastroschisis was reported as 1.76 per 10,000 births. [6] The gastroschisis rate increased from 1 in 3,600 in the years 1994-2000 to 1 in 1,667 in the years 2000-2002. [7]
Similarly, in 970 patients with gastroschisis, the prevalence was 0.131 per 10,000 in 1975 and 0.467 per 10,000 in 1996-1997. [8] The prevalence reported elsewhere is 0.46 per 10,000 [6] and a rate of increase by a factor of 10 and 20 fold has been reported. [9]
Low maternal age is associated with gastroschisis. [5,6] In some studies, 33% of infants with gastroschisis were born to mothers younger than 25 years of age. [10]
Classification
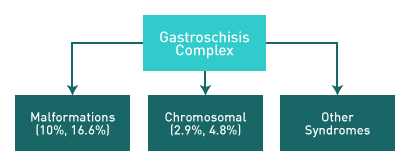
Above. Two classifications of gastroschisis have been proposed: “Simple” and “Complex.”
Simple gastroschisis suggests no other abnormalities while complex gastroschisis suggests gastrointestinal or other fetal anomalies. [11],[12]
Above. Complex gastroschisis is reported to have an association with syndromes or with malformations in a range of 10% to 16.6%, while the chromosomal abnormality rate is low. [10,11]
Etiology
Above. A number of potential theories have been proposed for congenital gastroschisis: 1) failure of mesoderm form in the anterior abdominal wall; 2) rupture of the amnion causing a herniation of the bowel; 3) abnormal involution of the right umbilical vein; 4) disruption of the right yolk sac artery with anterior abdominal wall damage; and 5) abnormal folding of the body wall resulting in an anterior defect. [13]
The defect may develop early during the third and fourth week after fertilization due to the failure of the endoderm and mesoderm to form the intestinal tube and the anterior abdominal wall. [14] Embryological folding towards the mid-line fusion may fail and result in the defect. [15] Gastroschisis may occur prior to abdominal wall closure as early as 35 days [16], while ischemia during the first trimester could play a role in its development. [17] The defect is usually to the right of the umbilicus, separated by a narrow strip of skin, and is usually a relatively small defect compared to the volume of herniated abdominal contents. [18]
Genetics
Gastroschisis is usually sporadic with some reports of familial cases. [19] Other reports also suggest that in families of infants with multiple defects, a sporadic or non-genetic mode is more likely. [18]
A change in paternity may suggest a maternal immunologic response to a new set of paternal antigens, which may alter the risk for gastroschisis. [20]

Above. The aneuploidy rate is lower for gastroschisis compared to omphalocele. About 3.0% of infants with gastroschisis have chromosomal abnormalities. (Study A) [21]
Elsewhere, chromosomal abnormalities are reported as follows: 4.8% for gastroschisis, 17% for omphalocele (Study B) [22], and 27.6% for omphalocele. (Study C) [23] Further, chromosomal abnormalities are reported in 2.9% of the gastroschisis group, and 20% for the omphalocele group. (Study D) [24]
In summary, the rate for karyotype abnormalities is low in gastroschisis and a rate of associated karyotype abnormalities for omphalocele is frequently quoted at about 30%.
Risk Factors
Above. Risk factors for gastroschisis include: low maternal age, caucasian descent, maternal smoking, and maternal recreational drug use. [25]
Above. The prevalence for gastroschisis is increased due to low maternal age. Low maternal age is also a factor related to an increased likelihood of complications such as premature birth. Decreasing prevalence is noted from Northern to Southern Europe. The defect is more common in Caucasians compared to Blacks or Asians. [26]
| Gastroschisis: Associated Non-bowel malformations |
| Cardiovascular |
| Limb defects |
| Central nervous system malformations |
| Renal malformations |
Above. Other potential risk factors are those which are associated with malformations.
Malformations that are not associated with the primary defect include congenital heart and limb defects [27] as well as central nervous system and renal defects. Associated malformations were noted in 16.6% of gastroschisis patients. [28] In a series of patients at Minneapolis Children’s Hospital, 17 of 155 (11%) of patients had a non-GI anomaly and 5 of 155 (3%) had a cardiac defect [29]
In other reports, congenital heart abnormalities have been reported in 4%, 6.8%, and 15% of infants with gastroschisis. [30],[31],[32] Pulmonary stenosis, supraventricular tachycardia, and peripheral pulmonary hypertension have been reported. [29]
Outcome
Above. In 159 pregnancies with gastroschisis, 42% of the mothers were less than 21 years of age. [33] In addition, those pregnancies were likely associated with premature delivery and low birth weight infants.
Teenage mothers (19 years or less) with gastroschisis pregnancies were twice as likely to give birth to low birth weight infants and three times as likely to have preterm infants compared to older mothers. [34]
Above. Preterm labor and delivery occurred in women less than 21 years of age at a rate of 28% compared to 6% for older women. Low birth weight babies were born to the younger mothers at a rate of 36% versus 10% for older mothers. [31]
Above. The “Simple” gastroschisis group gave birth later at 37.5 weeks compared to the “Complex” gastroschisis group which gave birth at 34 weeks. [35]
Simple gastroschisis group: No other congenital abnormalities and no associated bowel defects.
Complex gastroschisis group: Associated malformations and/or bowel complications.
Above. The overall infant survival rate with gastroschisis is over 92% in modern tertiary care centers. [36] Some centers report an overall survival rate of 97.2%. [37] However, survival rates decrease (48%) in regions with poor prenatal diagnosis and high numbers of outborn infants. [38]
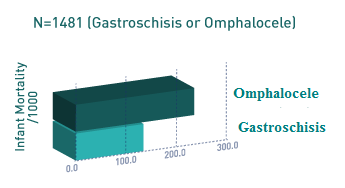
Above. The infant mortality rate for gastroschisis is 118 per 1000 which is lower than the infant mortality rate for omphalocele (215 per 1000). [39] Black infants with gastroschisis have a worse outcome compared to Caucasians, but Black infants with omphalocele have better survival rates compared to Caucasian or Hispanic groups.
The outcome for fetuses with gastroschisis is dependent upon the bowel status and ultrasound detection of fetal bowel dilatation is a predictor of neonatal bowel atresia. [40] Utilizing a temporary ileostomy before definitive repair, survival with bowel atresia is common.
While 25 mm or more of small bowel dilatation increased the risk for short term complications. [41], dilatation of 18 mm or greater did not always predict a difference in outcome. [42]
Above. Poor outcome parameters for gastroschisis are listed.
In one study of 64 gastroschisis patients, 47% had at least one GI abnormality, such as bowel dilatation, echogenic bowel, mattered bowel, herniation of stomach, and loss of bowel peristalsis, but these isolated findings did not correlate with several outcome measures. [43]
Fetal growth restriction in gastroschisis does not always affect outcome [44], while polyhydramnios may be an important finding for neonatal outcome since the presence of polyhydramnios is associated with bowel complications. [45]
Sonographic Detection of Growth Restriction in Gastroschisis.
A retrospective cohort study was performed assessing 111 births with gastroschisis. [46] Growth restriction was diagnosed using Hadlock’s nomogram and was defined as an estimated fetal weight of less than the 10th percentile for gestational age. The sensitivity and negative predictive value for the sonographic prediction of the small for gestational age neonate was 90% by 32 weeks and approximately 95% after 32 weeks. A low birth weight percentile on the antenatal scan was not associated with an immediate increase in neonatal morbidity or mortality.
Depending upon the health care system, antenatal detection of gastroschisis may not change the timing of surgery, frequency of complications, surgical outcomes, or length of stay.
Management
Elective preterm delivery in patients with gastroschisis is controversial. A recent Cochrane review regarding preterm birth for infants with gastroschisis was not able to define whether preterm intervention was beneficial. [47] However, recent Canadian experience suggests that induction of labor at 37 weeks was associated with less sepsis, bowel damage, and neonatal death compared to pregnancies managed beyond 37 weeks. [48]
Route of Delivery for Infants with Gastroschisis
Mode of delivery is not associated with neonatal survival for infants with gastroschisis. In 354 patients with gastroschisis, 174 of whom delivered vaginally and 180 by caesarean section, preterm birth rather than small for gestational age was the best predictor for neonatal death. [49]
The risk of neonatal death tends to be the same whether delivery is by vaginal or by cesarean section and outcomes following vaginal delivery were similar to those after caesarean section. [50]
Cesarean section does not improve the outcome in cases of major malformations such as fetal gastroschisis. [51] The routine use of caesarean section for gastroschisis delivery is not supported by other reviews. [52]
Fetal Testing of the Abdomen
Above. The rationale for fetal testing in gastroschisis is based upon evidence of intrauterine death and fetal growth restriction.
A program of fetal surveillance included every 4th-week assessment of bowel dilatation, thickness, motility, amniotic fluid volume, and fetal growth. [53]
Twenty-two percent of infants with gastroschisis had abnormal fetal heart tracings. [54] Initiation of antenatal testing by 28 to 29 weeks is proposed and resulted in no intrauterine fetal deaths. [55]
No randomized trials specify the type or frequency of testing. Given the likelihood of intrauterine death, fetal growth restriction, and the potential prognostic value of amniotic fluid volume, in the absence of complicating factors, weekly biophysical profile testing seems reasonable.
Gastroschisis: Imaging Considerations
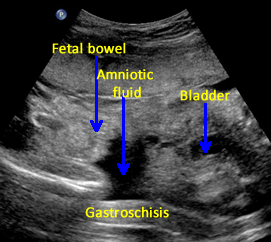
The following are ultrasound criteria for the diagnosis of gastroschisis:
• Small bowel present in amniotic fluid.
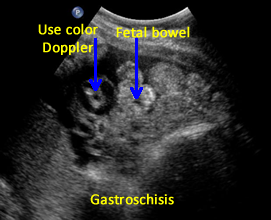
• Umbilical cord inserts normally (Use Color Doppler).
• No covering membrane.
Above. About 10% of infants with gastroschisis have other malformations. [56] Other large series (334,262) report slightly higher malformation rates of 16.6% [57] Overall, about 50% of the patients with gastroschisis had malformations related to the abdominal wall defect while half of the group had defects not associated with gastroschisis. [1]
Above. GGastroschisis may also be considered as multiple congenital anomalies (MCA). Four hundred and sixty-nine non-isolated cases of gastroschisis were registered in one study. [58] There were 41 chromosomal syndromes, 24 other syndromes, and 404 with multiple congenital anomalies (MCA). [3]
Among those with multiple congenital anomalies, four groups were most frequent: 1) Central nervous system, 4.5%; 2) Cardiovascular system, 2.5%; 3) limb abnormalities, 2.2%; and 4) kidney anomalies, 1.9%. [3]
Above. Other malformations specifically related to gastroschisis include: 1) malrotation; 2) cryptorchidism; 3) amyoplasia; 4) urinary tract obstruction; and intestinal atresia or stenosis [59]
Above. Ultrasound findings related to prognosis for gastroschisis include gastric dilatation, other bowel dilatation, polyhydramnios, fetal growth restriction, and other malformations.
In 34 infants with gastroschisis, 21 had no gastric dilatation and 13 had gastric dilatation. Those with gastric dilatation had a higher probability for neonatal death, volvulus, and prolonged hospital care. [60] Both gastric dilatation and large defect are important predictors of outcome. [61]
Abnormal dilatation of fetal bowel of 6 mm to 35 mm was associated with slower oral feeding, decreased primary closure, and increased readmission for bowel obstruction. [62]
Some reports consider the presence of intestinal atresia to be the single most important finding in those with poor outcomes. [63]
Magnetic resonance imaging (MRI) provides a potentially improved contrast without the risk of radiation effect and has been used in anomalies such as congenital diaphragmatic hernia and gastroschisis. [6]
The possibility of 3-D mode in MRI makes the assessment of fetal bowel condition potentially more accurate.
Gastroschisis: Images
Above. Gastroschisis. Case 1. Twin gestation. 11 weeks. Upper image: Normal Twin A. Lower image: Abnormal Twin B with increased nuchal translucency and anterior abdominal wall defect with herniation of bowel without apparent covering membrane.
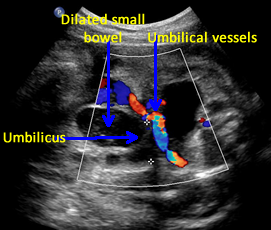

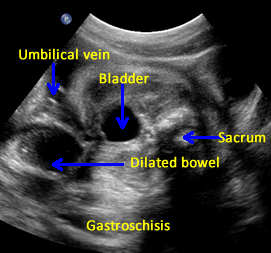
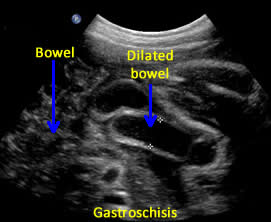
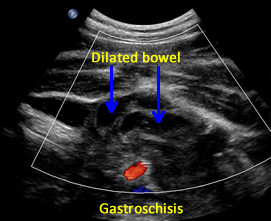
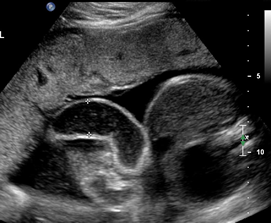
Above. Gastroschisis. Case 2. 30 3/7 weeks gestation. Axial ultrasound with normal umbilical cord insertion into umbilicus and dilated herniated fetal bowel.
Above. Gastroschisis. Case 2. 30 3/7 weeks gestation. Oblique view of the fetal pelvis demonstrating the relationship between the dilated bowel and fetal pelvic anatomy.
Above. Gastroschisis. Case 2. 30 3/7 weeks gestation. All images above demonstrate significant fetal bowel dilatation. Delivery occurred at 31 3/7 weeks. Decreased fetal movement for 3 days. Biophysical profile score of 2 of 10. Apgar scores of 4 and 8. Neonatal survival following Silo placement and subsequent neonatal surgery.
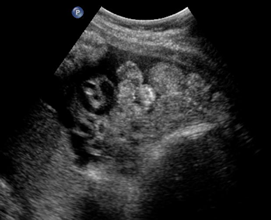
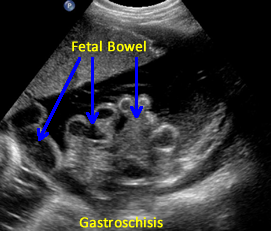
Above. Gastroschisis. Case 3. 20 5/7 weeks gestation. Normal umbilical cord insertion. Anterior abdominal wall defect. Note displacement of the fetal stomach. Lower images: Note echogenic rings typical of undilated small bowel and lack of membrane covering over bowel.
Above. Gastroschisis. Case 4. 26 4/7 weeks. Transverse of abdomen. Note displacement of the fetal stomach. Bowel demonstrates echogenicity, but few typical echogenic rings.
Above. Gastroschisis. Case 4. 26 4/7 weeks. A few echogenic rings are evident. Note absence of membranous covering for fetal bowel.
Above. Gastroschisis. Case 4. 26 4/7 weeks. Only a small amount of fetal bowel is evident.
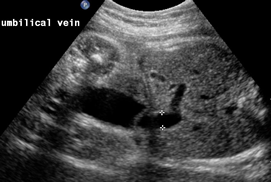
Above. Gastroschisis. Case 5. 30 1/7 weeks gestation. Gastroschisis with distortion and enlargement of the umbilical vein (9 mm).


Above. Gastroschisis. Case 5. 30 1/7 weeks gestation. Color Doppler is needed to distinguish umbilical cord vessels.
Above. Gastroschisis. Case 5. 30 1/7 weeks gestation. Typical fetal herniated small bowel without membranous covering. Color Doppler is needed to distinguish umbilical cord vessels.
Above. Gastroschisis. Case 5. 30 1/7 weeks gestation. An example of thickened, matted fetal bowel wall. Vaginal delivery occurred at 38 6/7 weeks. Early neonatal repair of the gastroschisis was accomplished without incident.
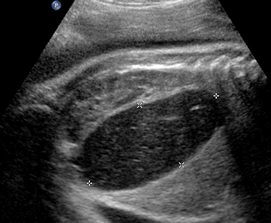
Above. Gastroschisis. Case 6. 34 3/7 weeks gestation. Large anterior abdominal wall defect.
Above. Gastroschisis. Case 6. 34 3/7 weeks gestation. Gastroschisis with enlarged fetal stomach and distorted hepatic vessels.
Above. Gastroschisis. Case 6. 34 3/7 weeks gestation. Dilated small bowel, polyhydramnios, and view of mesentery.
Above. Gastroschisis. Case 6. 34 3/7 weeks gestation. Fetal bowel dilatation. Spontaneous vaginal delivery at 38 weeks. 3300 gram infant with successful early gastroschisis neonatal repair.
Above. Gastroschisis. Case 7. 34 4/7 weeks gestation. Typical bowel findings in gastroschisis. Some echogenic rings are present, and there is increased amniotic fluid volume. Note absent fetal bowel membranous covering.
Above. Gastroschisis. Case 7. 34 4/7 weeks gestation. Transverse views of fetal abdomen with dilated fetal stomach and dilated bowel with an increase in amniotic fluid volume.
Above. Gastroschisis. Case 7. 34 4/7 weeks gestation. Fetal stomach is massively enlarged secondary to gastroschisis.
Above. Gastroschisis. Case 7. 34 4/7 weeks gestation. Fetal stomach is enlarged and there is dilatation of intra-abdominal bowel. Preterm labor and delivery occurred at 34 4/7 weeks. Neonatal repair of gastroschisis was successful.
Gastroschisis: Video
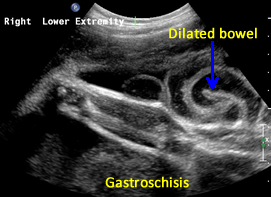
Above. Gastroschisis. Case 1. 26 4/7 weeks gestation. Herniated bowel in amniotic fluid. Intra-abdominal bowel is dilated.
Above. Gastroschisis. Case 1. 26 4/7 weeks gestation. Herniated bowel is typical for gastroschisis. Again, intra-abdominal bowel is dilated.
Above. Gastroschisis. Case 2. 30 1/7 weeks gestation. Typical herniated bowel within amniotic fluid. Note no covering membrane.
Above. Gastroschisis. Case 2. 30 1/7 weeks gestation. Note anterior abdominal wall defect with dilated fetal bowel.
Above. Gastroschisis. Case 3. 34.3 weeks gestation. Massively dilated fetal small bowel.
Above. Gastroschisis. Case 4. 30 3/7 weeks gestation. Umbilical cord insertion site with herniating fetal bowel.
Above. Gastroschisis. Case 4. 30 3/7 weeks gestation. Markedly dilated fetal bowel.
Above. Gastroschisis. Case 4. 30 3/7 weeks gestation. Gastroschisis with extensive dilatation of small bowel extending to lower extremities.
Omphalocele
Page Links: Definition, Prevalence, Classification, Risk Factors, Genetics, Management, Differential Diagnosis, References
Definition
Omphalocele is an abdominal wall defect in which intra-abdominal contents herniate outside of the abdomen at the umbilicus. The herniated organs, which can include fetal bowel, liver, and spleen, are covered by a sac that is composed of amnion and peritoneum. Associated problems, including karyotype abnormalities, are frequent and may affect outcome. [64],[65]
There is a normal physiologic herniation of the fetal bowel at the level of the umbilicus measuring 0.5 cm to 1.0 cm that can be observed as an echogenic mass from 7 to 10 weeks gestation and is no longer seen after 12 weeks gestation. [66] By the fourth week of gestation, the primitive gut begins to develop. Mid-line folding of the embryo results in the fusion of the abdominal wall. When fusion fails to occur, an abdominal defect develops and the abdominal organs protrude into the amniotic cavity. The resultant defect may be covered or uncovered.
Prevalence
The prevalence rate for omphalocele is reported at 1.6 per 10,000. [67] In general, the prevalence rate for anterior abdominal wall defects is increasing, but is greater for gastroschisis than for omphalocele. [68]
In Japan, the incidence of omphalocele was 0.322 per 10,000 births in 1975 to 1980 and 0.626 per 10,000 births in 1996 to 1997. [69] The prevalence rate varies by gestational age and by maternal age. [70] The prevalence of omphalocele in a population with the maternal age distribution of all deliveries in England and Wales was 7.4 per 10,000 at 12 weeks of gestation, and 3.5 per 10,000 at 20 weeks, and 2.9 per 10,000 in live births.
Classification
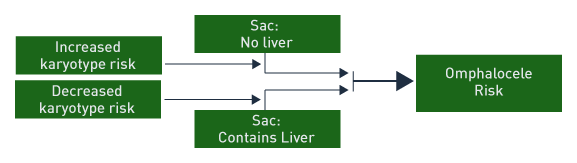
When the omphalocele sac contains only bowel and there are associated anomalies, high risk is present for chromosomal abnormality and poor outcome. When the omphalocele sac contains only the liver, the outcome is generally good with a low risk of chromosomal abnormality. [71]
Small omphalocele size correlates with an increase in gastrointestinal anomalies, but a lower risk for cardiac anomalies. [72]
Risk Factors
Above. Risk factors and associations with an omphalocele can be broadly categorized into 4 entities. Multiple congenital anomalies (non-syndromic), karyotype abnormalities, malformation complexes, and non-chromosomal syndromes. [73],[74]
Risk Factors for Omphalocele
Associated malformations (74.1 %).
Abnormal karyotype (29.3%).
Recognizable syndrome or an unspecified malformation pattern (44.8%).
Oligohydramnios.
Polyhydramnios.
The above risk factors and their relative frequency have been identified for omphalocele. [10]
Non-chromosomal Syndromes Associated with Omphalocele
Beckwith-Wiedemann syndrome.
Goltz syndrome.
Marshall-Smith syndrome.
Meckel-Gruber syndrome.
Oto-palato-digital type II syndrome.
CHARGE syndrome.
Fetal valproate syndrome.
Malformation Sequences Associated with Omphalocele
Ectopia Cordis.
Body stalk anomaly.
Exstrophy of the bladder.
Exstrophy of the cloaca.
OEIS (Omphalocele, Exstrophy of bladder, Imperforate anus, Spinal defect).
Malformation Complexes Associated with Omphalocele
Pentalogy of Cantrell.
Multiple Congenital Anomalies (MCA) Associated with Omphalocele (30.2%)
Musculoskeletal system (23.5%).
Urogenital system (20.4%).
Cardiovascular system (15.1%).
Central nervous system (9.1%).
These tables suggest associations and risk for omphalocele and the relative frequency of associations of MCA with omphalocele. [11]
Genetics
Among 256,858 births, 29.3 % of fetuses with omphalocele had an abnormal karyotype. [75] Other studies report similarly high abnormal karyotype rates of 17% and 20%. [76],[77] However, the rate of chromosomal aneuploidy is reported higher (8 of 17 or 47%) when determined between 11 and 16 weeks. [78] In addition, if the sac contained the liver, the karyotype was normal in this study. [15] Abnormal nuchal translucency and small sac size suggested a higher risk for abnormal karyotype. [15]
Among 15,726 viable, singleton pregnancies at 11-14 weeks of gestation, omphalocele was diagnosed in 0.11% of the cases. The combined frequency of trisomy 18, trisomy 13, and/or triploidy was 61%. The corresponding frequencies of omphalocele for fetuses was as follows:
Trisomy 18, 22.5%.
Trisomy 13, 9.1%.
Triploidy, 12.5% [79]
The prevalence of omphalocele among a population depends upon maternal age and gestational age at the time of diagnosis. [16]
Outcome
Survival rates with omphalocele depend upon the presence of associated chromosomal abnormalities or associated malformations. The most frequent karyotype abnormality is trisomy 18.
Survival
When chromosomal abnormalities are excluded, the survival rate for isolated omphalocele is reported at 44% (7/16). [80]
Associated Malformations
Irrespective of the karyotype, most series report a high rate of associated malformations: 26/33 (78%), 96/127 (76%), and 21/26 (81%), [81],[82],[83] and outcome is related to the type of anomaly. [84]
Other Factors Related to Outcome
Other factors related to outcome in fetal omphalocele:
Small sac size (higher prevalence of gastrointestinal anomalies). [85]
Premature delivery (14 of 27 fetuses or 52%). [21]
Fetal growth restriction (4 of 11 or 36%). [21]
Contents of sac: Liver and bowel versus bowel only. [86]
Liver and bowel (Associated anomalies, 7 of 20 or 35%).
Bowel only (Associated anomalies, 17 of 19 or 93%).
Liver and bowel (Chromosomal abnormalities, 1 of 17 tested or 6%).
Bowel only (Chromosomal abnormalities, 17 of 19 or 93%).
Liver and bowel (Survival, 14 of 39 or 36%).
Bowel only (Survival, 1 of 19 or 5%).
Management
Prenatal Management
Prenatal management involves serial assessment of the fetus for diagnosis, follow-up, and testing. [87] Amniocentesis for karyotype is indicated. Given the possibility of associated cardiac malformation and fetal growth restriction; targeted scan, cardiac echo, and follow-up scans for growth are indicated while fetal testing with biophysical profile on a weekly basis seems warranted. Delivery at a tertiary care center with a multi-disciplinary team is optimum. Route of delivery with small omphalocele does not alter outcome. [88]
Neonatal
Neonatal surgical management of these defects is not uniform or standardized. [89] Obvious variables in the success of surgical repair depend upon the defect size, the contents of the sac, the presence of karyotype abnormalities, and the type and extent of associated malformations. Primary closure versus delayed closure is an initial surgical decision. If closure is delayed due to the defect size, a variety of techniques are reported which include the use of synthetic versus biomaterial patches. [29] The latter include solvent-dried dura biomaterial grafts which promote rapid scar formation, and integration with adjacent skin tissue with the effect of reduced foreign body reactions at the site of implantation. [90] Other techniques include the use of prosthetic mesh, and silastic sacs, but small bowel obstruction during the first year of life occurs and can be fatal irrespective of the method chosen. [91]
Neonatal Management of Giant Omphalocele
Giant omphaloceles are defined as a liver containing protrusion through an abdominal wall defect which is wider than 5 cm. [92] These lesions are less likely to be associated with karyotype abnormality, but their size presents a surgical challenge and complications are reported such as liver infarction and respiratory insufficiency. [32],[93] Prolonged ventilator support may be necessary. [94]
Specific surgical approaches and techniques for closure are:
Intraperitoneal tissue expander (IPTE) to create adequate abdominal domain. [95]
Progressive stretching of the abdominal wall and enlargement of the abdominal domain. [96]
Tissue expanders positioned in the potential space between the internal oblique and transversus abdominis layers. [97],[98]
Component separation technique. [99]
Sequential sac ligation. [100]
Translation of the muscular layers. [101]
Vacuum-assisted closure. [102]
Bilateral longitudinal fibroperitoneal-aponeurotic transposition. [103]
In addition to the above, specific approaches to allow secondary closure and delay primary repair are as follows:
Absorbable mesh followed by split-thickness skin graft. [104]
Synthetic mesh plus povidone-iodine solution. (Betadine) [105]
Human acellular dermis. [106],[107],[108]
Silver sulfadiazine dressing changes. [109]
Absorbable mesh and skin flaps or grafts. [110]
Bovine pericard patch. [111]
Differential Diagnosis
Omphalocele: Imaging Considerations
Ultrasound Appearance
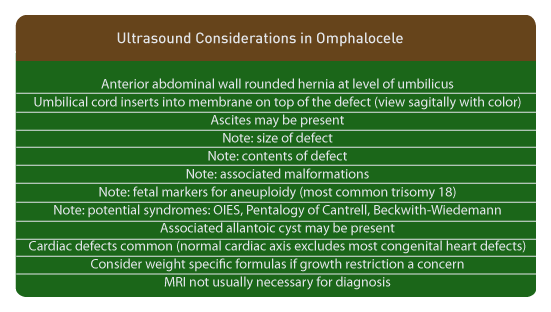
Above. The mid-trimester ultrasound appearance of omphalocele depends upon the size of the defect, the contents within the hernia, the presence of ascites, and the presence of other malformations. [112] The defect herniates through the umbilicus, is covered by a 2 layer membrane, and the umbilical cord inserts apically on the sac. Ascites may be present within the sac. The cord insertion is best viewed sagittally and color Doppler can be used to follow the umbilical vein through the sac. The presence or absence of the liver within the sac should be noted since small defects with echogenic bowel are more likely to be associated with abnormal karyotype. [1] The presence of anomalies, especially those associated with karyotype abnormalities, should be noted. There is no free floating bowel outside of the hernia sac.
Early diagnosis at 12 to 16 weeks gestation is possible with transvaginal ultrasound. A relatively high rate of associated anomalies is reported (22 of 38 or 58%) in addition to a relatively high frequency of aneuploidy among those with anomalies. [113] A small omphalocele, detected at 12 to 16 weeks in a fetus with a normal karyotype, may disappear by 20 to 24 weeks.
A normal cardiac axis tends to exclude congenital heart defects in fetuses with omphalocele. [114]
In fetuses with giant omphaloceles, pulmonary hypoplasia may be present. The lung/thorax transverse area ratio (L/T) and chest/trunk length ratio (C/T) is reported to be useful in predicting pulmonary hypoplasia. [115]
Allantoic cysts of the umbilical cord may be associated with omphalocele, as well as with aneuploidies. [116]
Since the abdominal circumference is artificially small in fetuses with omphalocele, specific weight formulas are useful and accurate. [117]
MRI may confirm findings on prenatal ultrasound, but is not usually necessary for diagnosis. [118]
Omphalocele: Images
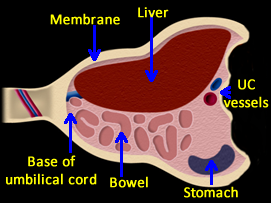
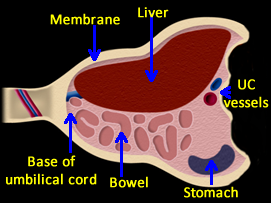
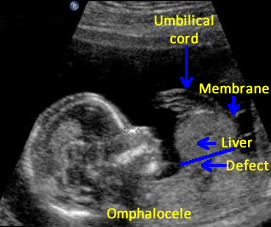
Above. Schematic of omphalocele. Abdominal contents are herniated into the base of the umbilical cord. Most often, liver and bowel are present in the sac, but the stomach may also be within the sac. Umbilical vessels insert onto the membrane covering.
Above. Omphalocele. Large omphalocele demonstrating intra-sac contents, and membrane covering. Umbilical cord on the right of the image inserts laterally onto the omphalocele membrane.
Above. Omphalocele. The liver is one of the principal organs present within the omphalocele sac. Note the homogeneity of the hepatic echos, which are distinct from the other intra-abdominal echos.
Above. Pseudo-omphalocele caused by oblique scan plane. Note lack of membrane covering, and the shape of the inferior vena cava, which also suggests an oblique scan plan. There is no distinct anterior abdominal defect. Pseudo-omphalocele can also be caused by excessive transducer pressure.
Above. Omphalocele. Case 1. 12 5/7 weeks gestation. Note apparent anterior wall defect.
Above. Omphalocele. Case 1. 12 5/7 weeks gestation. Longitudinal view suggests membranous covering of the mass suggesting a diagnosis of omphalocele.
Above. Omphalocele. Case 1. 12 and 5/7 weeks gestation. Transverse views confirm likely anterior abdominal defect and omphalocele.
Above. Omphalocele. Case 1. 12 5/7 weeks gestation. Umbilical cord insertion onto omphalocele membrane.
Above. Omphalocele. Case 2. 15 weeks gestation. Longitudinal view. Note anterior wall defect with herniation of liver. Also note membranous sac with umbilical cord insertion.
Above. Omphalocele. Case 2. 15 weeks gestation. Transverse view. The defect is demonstrated. Note covering membrane and umbilical cord relationships with the mass.
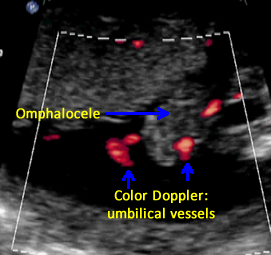
Above. Omphalocele. Case 2. 15 weeks gestation. Upper images of Color Doppler demonstrate umbilical cord insertion onto the omphalocele. Lower images demonstrate 2 umbilical arteries as they exit from the fetal abdomen.
Above. Omphalocele. Case 3. 20 6/7 weeks. Normal karyotype. Membrane is visible as well as the umbilical cord insertion onto the membrane. The liver is within the omphalocele sac.
Above. Omphalocele. Case 4. 23 weeks gestation. Two umbilical arteries are identified exiting from the fetal abdomen. An omphalocele containing liver is on the right side of the image.
Above. Omphalocele. Case 4. 23 weeks gestation. Liver is within the sac and the hepatic vessels are demonstrated by color Doppler.
Above. Omphalocele. Case 5. Mid-trimester omphalocele with stomach entering the omphalocele sac.
Above. Omphalocele. Case 6. Mid-trimester omphalocele with liver, membrane, and hepatic vessels.
Above. Omphalocele. Case 7. 20 week scan. Omphalocele with umbilical cord relationships.
Omphalocele: Video
Above. Large omphalocele. Note membrane. Umbilical cord
as demonstrated with color Doppler inserts on covering membrane.
Above. Umbilical cord insertion as demonstrated by color Doppler.
Above. Note large omphalocele sac and membrane. Liver is within
the omphalocele.
Above. Transverse omphalocele sac membrane and liver within
the omphalocele.
Above. The abdomen is to the left side of the image. The stomach is
partially within the omphalocele.
Above. Omphalocele with umbilical cord insertion onto
the covering membrane.
Above. Omphalocele with abdomen on the left side of the image
and the stomach remains within the abdomen.
Above. Color Doppler in initial frames and color power Doppler
in later frames showing umbilical cord insertion onto
omphalocele at 17 weeks gestation.
References
Prenatal diagnosis of anterior abdominal wall defects: Pictorial essay. Agarwal R. Indian J Radiol Imaging. 2005; 15: 361-372. Abdominal wall defects. Aspelund G, Langer J. Current Paediatrics. 2006; 16 (3): 192-8. Prenatal diagnosis of anterior abdominal wall defects: Pictorial essay. Agarwal R. Indian J Radiol Imaging. 2005; 15: 361-372. -
Abstract: PMID: 18551719 -
Abstract: PMID: 15266212 -
Abstract: PMID: 11755106 -
Abstract: PMID: 15280125 -
Abstract: PMID: 10646777 -
Abstract: PMID: 18655097 -
Abstract: PMID: 18204766 -
Abstract: PMID: 17992713 -
Abstract: PMID: 11150437 -
Abstract: PMID: 17230493 -
Abstract: PMID: 18655098 -
Abstract: PMID: 18655098 -
Abstract: PMID: 18338390 -
Abstract: PMID: 17301669 Gastroschisis: embryology, pathogenesis, epidemiology. Chabra S, Gleason CA. NeoReviews. 2005;6(11):e493. Neoreviews. Vol. 14 No. 8. August 2013. -
Abstract: PMID: 17163540 -
Abstract: PMID: 10646777 -
Abstract: PMID: 18204766 Characteristics and perinatal course of prenatally diagnosed fetal abdominal wall defects managed in a tertiary center in Japan. Hidaka N, Murata M, Yumoto Y, Hojo S, Fujita Y, Masumoto K, Taguchi T, et al. Journal of Obstetrics and Gynecology Research. 2008 Aug;35(1):40-7. -
Abstract: PMID: 15754571 -
Abstract: PMID: 18551719 -
Abstract: PMID: 18655097 -
Abstract: PMID: 18655099 -
Abstract: PMID: 18386803 -
Abstract: PMID: 19433170 -
Abstract: PMID: 16157146 -
Abstract: PMID: 12704739 -
Abstract: PMID: 17766615 -
Abstract: PMID: 18022429 -
Abstract: PMID: 16240382 -
Abstract: PMID: 11150437 -
Abstract: PMID: 14703785 -
Abstract: PMID: 12415370 -
Abstract: PMID: 11283877 -
Abstract: PMID: 15368557 -
Abstract: PMID: 17029299 -
Abstract: PMID: 18186152 -
Abstract: PMID: 18206451 -
Abstract: PMID: 18405710 -
Abstract: PMID: 15300527 -
Abstract: PMID: 12704738 -
Abstract: PMID: 26518276 -
Abstract: PMID: 23737031 -
Abstract: PMID: 23635735 -
Abstract: PMID: 15458885 -
Abstract: PMID: 17985136 -
Abstract: PMID: 15758605 -
Abstract: PMID: 15137010 -
Abstract: PMID: 15793730 -
Abstract: PMID: 14970991 -
Abstract: PMID: 18538153 -
Abstract: PMID: 18655099 -
Abstract: PMID: 18386803 -
Abstract: PMID: 17357116 -
Abstract: PMID: 18386803 -
Abstract: PMID: 15167837 -
Abstract: PMID: 17766615 -
Abstract: PMID: 16677878 -
Abstract: PMID: 18068889 Abdominal wall defects. Aspelund G, Langer J. Current Paediatrics. 2006;16(3):192-8. Prenatal diagnosis of anterior abdominal wall defects: pictorial essay. Agarwal R. Indian J Radiol Imaging. 2005;15:361-372. -
Abstract: PMID: 2945223 -
Abstract: PMID: 8475457 -
Abstract: PMID: 15280125 -
Abstract: PMID: 10646777 -
Abstract: PMID: 8590187 Outcome of fetal omphalocele according to omphalocele content combined with the associated anomaly. Jung SI, Cho JY, Ryu JK. J Korean Soc Med Ultrasound. 2005 Jun;24(2):61-6. -
Abstract: PMID: 19040938 -
Abstract: PMID: 11755106 -
Abstract: PMID: 18386803 -
Abstract: PMID: 11755106 -
Abstract: PMID: 18204766 -
Abstract: PMID: 15754571 -
Abstract: PMID: 9203209 -
Abstract: PMID: 8590187 -
Abstract: PMID: 17674424 -
Abstract: PMID: 19215546 -
Abstract: PMID: 15266212 -
Abstract: PMID: 17985136 -
Abstract: PMID: 16580922 -
Abstract: PMID: 19040938 Outcome of fetal omphalocele according to omphalocele content combined with associated anomaly. Jung SI, Cho JY, Ryu JK. J Korean Soc Med Ultrasound. 2005 Jun;24(2):61-6. -
Abstract: PMID: 18634119 -
Abstract: PMID: 11704185 -
Abstract: PMID: 18414971 -
Abstract: PMID: 12152643 -
Abstract: PMID: 18358285 -
Abstract: PMID: 16308903 Challenges of giant omphalocele: from fetal diagnosis to follow-up. Davis AS, Blumenfeld Y, Rubesova E, Abrajano C, El-Sayed YY, Dutta S, Barth RA, et al. NeoReviews. 2008;9(8):e338. Link: http://neoreviews.aappublications.org/cgi/content/abstract/neoreviews;9/8/e338
-
Abstract: PMID: 17323674 -
Abstract: PMID: 16567180 -
Abstract: PMID: 15795823 -
Abstract: PMID: 15065039 -
Abstract: PMID: 15544772 -
Abstract: PMID: 18206491 -
Abstract: PMID: 16865514 -
Abstract: PMID: 15803336 -
Abstract: PMID: 16410135 -
Abstract: PMID: 15213910 -
Abstract: PMID: 17043872 -
Abstract: PMID: 16490981 -
Abstract: PMID: 16516614 -
Abstract: PMID: 16410136 -
Abstract: PMID: 15300551 -
Abstract: PMID: 17101356 -
Abstract: PMID: 12720180 -
Abstract: PMID: 17628735 Ventral wall defects. Hertzberg BS, Nyberg DA, and Neilsen IR. In: Diagnostic imaging of fetal anomalies. Nyberg DA, McGahan JP, Pretorius DH, and Pilu G. (eds). Lippincott, Williams, and Wilkings, Philadelphia 2003. pp 519-539. -
Abstract: PMID: 15220502 -
Abstract: PMID: 1693335 -
Abstract: PMID: 17960394 Isolated umbilical cord cyst: a case report. Cabrita B, Cadilla J, Gonçalves L, Lilaia C, Ribeiro dos Santos C, Santos C. The Internet Journal of Gynecology and Obstetrics. 2007;7(1). -
Abstract: PMID: 18383477 -
Abstract: PMID: 12403388
1
2
3
18
19
23
64
65
71
86
93
112
116